Information for PIs and Research Personnel
- Principal Investigator (PI) and Research Personnel Roles & Responsibilities
-
PI and Researcher responsibilities (summarized from Policy 49):
1. PIs and research personnel are responsible to fulfill the responsibilities described in PPMs 290-27, 290-55, 290-56, and 290-75. 2. All researchers utilizing animal care spaces at UC Davis must be included on the Principal Investigators lab roster maintained in the online Safety Suite and complete UC Laboratory Safety Fundamentals course (initial) or UC Laboratory Safety Refresher (every 3 years after initial). 3. Researchers using hazardous materials and/or Standard of Care medications in their research laboratories are also responsible for following campus policies regarding use of these materials in their laboratories. Researchers should consult with their respective Department Safety Coordinator (DSC) and/or Environmental Health & Safety (EH&S) to ensure applicable policies are followed. 4. All materials administered to animals as part of a research or teaching exercise, including materials believed to be non-hazardous and Standard of Care medications, must be listed in the approved Animal Care and Use Protocol in Sections 14a and 14b. Animal care spaces and support areas in the vivarium are considered an extension of the laboratory space and all safety measures for labelling, use and transport still apply. 5. List all hazardous materials which will be used in Section 8, as well as 14a and 14b of the Animal Care and Use Protocol. Clearly identify the type of hazardous material(s) being used, where it is being used, and if the material poses a potential risk to individuals or other animals in the vivarium.
-
- Note: If a Standard of Care medication is being administered outside of a veterinary formulary approved route or being administered via a non-standard method (e.g., water-bottle) and has known hazards, it must be included in Section 8 and it may require a VHSS.
6. Comply with any special handling indicated for the materials covered in the approved protocol. 7. Provide training to lab members regarding specific animal handling techniques or processes related to the protocol. This must include appropriate containment practices, spill clean-up, and waste disposal requirements. Consult with the Facility Manager or Technician-in-Charge for vivarium-specific practices. 8. Prior to using a hazardous material or Standard of Care medication, either within the vivarium space or with the animals, the PI, or designee, must notify the Facility Manager/Technician-in-Charge with the established facility defined method. Indicate each time animals are dosed (hazardous materials) using the methods outlined by the specific Facility (e.g., door sign, hazard card) including date, material, dose, route, and clearance date, if applicable. 9. If a material is determined to be an environmental hazard but does not pose a health risk to animal care staff, or if a material has specific disposal requirements, a VHSS will be created to ensure proper handling. The PI is responsible for indicating when animals are dosed using the notification method outlined by the Facility. 10. Label cage cards/cages with appropriate hazard symbol (as applicable according to the VHSS) and/or the facility defined method. 11. The PI/researcher is responsible for the proper handling and disposal of any materials indicated in the protocol and/or VHSS, such as contaminated water or food. 12. Hazardous materials for animal use must be prepared in the laboratory. Only transport the necessary amount to the vivarium for immediate use (see SafetyNet #150 for more information on how to transport hazardous materials safely). Prior approval from the Facility Manager/Technician-in-Charge must be obtained to prepare or store any hazardous materials in the vivarium. 13. Hazardous materials must only be administered in approved locations within the vivarium. Review the VHSS and/or consult with the Facility Manager/Technician-in-Charge to determine these locations. 14. Hazardous materials and Standard of Care medications cannot be stored in the vivarium without prior authorization from the Facility Manager/Technician-in-Charge. Once approved, storage must be consistent with SafetyNet #42 General Guidelines for Storage and Management of Laboratory Chemicals and the Laboratory Safety Manual. Storage of any hazardous chemicals or Standard of Care medications in the vivarium must be included in the online chemical inventory of the PI. 15. Ensure the safe use of hazardous materials and Standard of Care medications, including appropriate disposal of research materials, and cleanup of any chemical or biohazardous or biological spills. 16. Alert Facility Manager/Technician-in-Charge of any vivarium-owned PPE that has become contaminated. Provide information on special handling or disposal of the contaminated PPE. -
- Training Requirements
- PIs and research personnel who use animal facilities are subject to training requirements and required documentation from research, as well as animal care. All researchers handling animals or administering substances to animals must be listed on the appropriate Animal Care and Use Protocol. Training required for the animal protocol must be completed prior to handling animals.
ResearchBe included on the PIs lab roster located in the UC Online Lab Safety Tools. Review the Lab Hazard Assessment (LHAT) and complete PPE training. Complete the UC Laboratory Safety Fundamentals course (initial) or UC Laboratory Safety Refresher (every 3 years after initial). Complete all required initial and renewal training of the home (PIs) laboratory. Review and complete training on any Standard Operating Procedures (SOPs) pertaining to the specific chemical or hazard class that will be used in the vivarium/as part of an animal study. Complete training associated with specific hazards including, but not limited to, required training for handling recombinant, biohazardous and radioactive materials. PIs must train all researchers included on an animal protocol on the specific procedures listed, including hazardous materials handling. Any additional training set forth by the PI or Facility Manager/Technician-in-Charge.
Animal Care
Required training is indicated on the Training Record Template. Consult with IACUC for any questions or concerns regarding required training before starting work with animals. - Animal Use and Care Approval Process when Hazardous Materials are Included
-
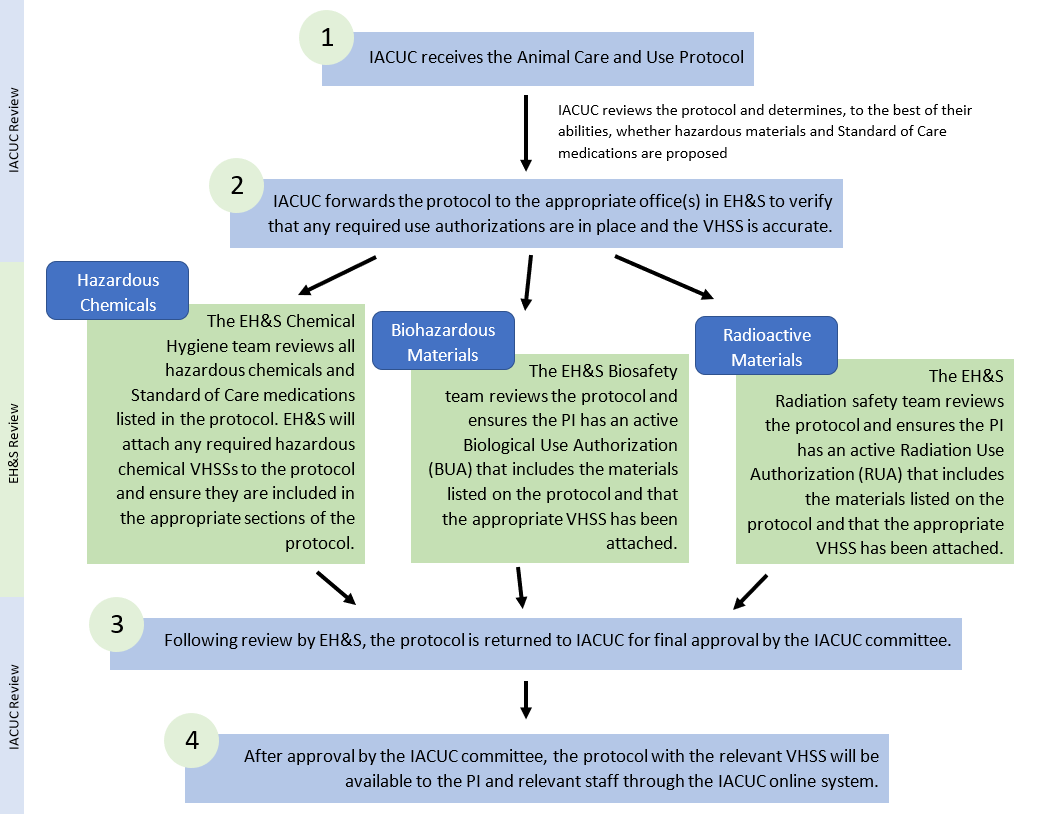
- Steps to Take Before Starting Animal Work with Hazardous Materials
-
1. The PI or research staff must notify the Facility Manager/Technician-in-Charge prior to initiating the component of the project with the hazardous material. 2. The Facility Manager/Technician-in-Charge will ensure animal care staff are aware of imminent studies and make Section 14b available to them. 3. The Facility Manager/Technician-in-Charge will use the information on the VHSS to create the appropriate animal room door posting, ensure staff have access to the relevant safety information, and provide training to animal care staff if they will be handling the animals and cages. 4. The PI must indicate each time animals are dosed (hazardous materials, as well as Standard of Care medications, and environmental hazards) using the methods outlined by the specific Facility, as well as labeling cage cards/cages with appropriate hazard symbol, as indicated on the VHSS. 5. It is the PIs responsibility to ensure hazardous materials and Standard of Care medications are prepared in the laboratory and only the necessary amount is safely transported to the vivarium for immediate use (see SafetyNet #150 for more information on how to transport hazardous materials safely). 6. The PI and research staff are responsible for safe use of hazardous materials and Standard of Care medications including appropriate disposal of research materials and cleanup of any chemical or biohazardous spills. Alert animal care staff of any vivarium-owned PPE that has become contaminated and provide information on proper handling/disposal. - Vivarium Hazard Safety Sheet (VHSS) Information
-
What is a VHSS?
Viviarum Hazard Safety Sheets (VHSSs) serve as a tool to inform animal care staff of specific hazards and to inform of proper administrative and engineering controls, PPE, and waste disposal requirements. A VHSS is created for protocols where animals will be administered hazardous chemicals, recombinant/biohazardous material, and radioactive material, as well as for environmental hazards or materials with non-routine disposal requirements to ensure proper handling.
Note: the investigator is ultimately responsible for ALL the hazards they introduce into the vivarium. Standard of Care medications such as analgesics and antibiotics are hazards that do not receive a VHSS, however, the PI is still responsible to ensure proper hazard communication to animal care staff using the method outlined by the facility.
Why do I, as a researcher, need to know about VHSSs?
The IACUC will not approve protocols that involve hazardous materials until the appropriate 'use authorization' or clearances are in place, and the corresponding VHSSs are attached to the protocol. These 'use authorizations' or clearances include:Biological Use Authorization (BUA) for projects involving recombinant nucleic acids, human tissue/cells/certain body fluids, viral vectors, or infectious agents. Radiation Use Authorization (RUA) for use of radioisotopes, Stem Cell Research Oversight Committee (SCRO) approval for work involving human stem cells, and/or Clearance by the Chemical Hygiene team for projects involving hazardous chemicals, including environmental hazards. It is important to realize that although you have direct knowledge of the hazards of the materials you use and interact with, animal care staff are reliant on us (PIs, researchers, IACUC, and EH&S together) to ensure they are informed of the hazards of the materials in their work area. VHSSs provide the means to communicate the handling requirements of hazardous materials to animal care staff in order to protect animal care staff when supporting our research endeavors.
How do VHSSs get attached to my protocol?
VHSSs for hazardous materials covered by a BUA or RUA can be attached to the protocol by the PI/researchers when the protocol is being filled out. Look for the appropriate VHSS in the IACUC VHSS library and select the appropriate VHSS to attach.
VHSSs for hazardous chemicals go through a separate process. At the time of submission, no VHSSs need to be attached, but all hazardous materials must be listed in Section 8, and all materials administered to animals in the study must be listed in Sections 14a and b. The Chemical Hygiene team reviews the use of the hazardous chemicals, develops a VHSS, and attaches the VHSS to the protocol.
And what do I do with them once they are attached?
Once the VHSS is attached to the protocol the PI/researcher must read and understand the VHSS and ensure it is posted appropriately. IACUC will inform the necessary animal care facility that the approved protocol has VHSSs attached. The facility manager/technician-in-charge may have additional questions for the PI/researcher regarding the hazardous material.
What hazardous chemicals receive a VHSS?
Hazardous chemicals that receive a VHSS are ones that can pose a health hazard to the animal care staff that may handle animals administered these materials or chemicals that pose a threat to the environment. Of particular emphasis are carcinogens (Globally Harmonised System of Classification and Labelling of Chemicals (GHS) hazard codes H350, H351), reproductive hazards (GHS hazard codes H340, H341, H360, H361), antineoplastics, chemicals with unknown hazards, and Schedule I Controlled Substances/hallucinogenic material, or any material whose hazards cannot be sufficiently addressed by the Facilities Hazard Communication Program. Environmental hazards (H400, H410) also receive a VHSS: in this case a VHSS is issued to ensure proper disposal of bedding to protect the environment.
How can I "speed up" the clearance process by the Chemical Hygiene team?
Providing the full name of the chemicals and drugs being used (e.g., 'azoxymethane' as opposed to 'AOM', 'clenoliximab' rather than 'anti-CD47'), as well as the Chemical Abstracts Service (CAS) number of the chemicals and drugs being used can help to speed up the review process. Alternatively, providing a copy or a link to the manufacturers’ Safety Data Sheet(s) can also help speed up the process.
My material didn't get a VHSS, that means it has no hazards, right?
Not quite. VHSSs are intended to provide animal care staff with additional information on how to handle contaminated animals and bedding safely using the rubric above. Hazardous materials that can be safely handled under the Facility's established Hazard Communication Program, such as Standard of Care medications, or those materials that do not pose any health hazard to animal care staff when handling contaminated animals or bedding, do not receive a VHSS. These materials may still be corrosive, flammable, acutely toxic, etc. Be prepared to share the hazards of any materials you may use in an animal care facility with animal care staff, Facility Managers/Technicians-In-Charge. -
Cage Card Examples
- Cage cards and animal records are utilized in most vivaria to communicate husbandry and exposure information to vivarium staff and researchers.
Biohazard Cage Card Example
Chemical Hazard Cage Card Example
Radiation Hazard Cage Card Example


