The transportation of clinical human specimens on public roads is considered materials of trade (MOTs).
The Department of Transportation’s Pipeline and Hazardous Materials Safety Administration provides a broad overview of what this means. Materials of Trade fall under CFR 173.6.
>> Download combination packaging image (.png)
Staff may use private vehicles to transport exempt human samples
They must adhere to the following criteria:
- These individuals must be adequately trained and made aware of the potential hazards contained within the packages (e.g Bloodborne Pathogen Awareness training).
- There should also be a plan in place to deal with a spill if one were to occur during transport.
- The vehicle used must be for direct and exclusive transport of the sample to the destination which means:
- No passengers
- No other cargo
- No unnecessary stops permitted
- Transporters must follow the UCOP Travel regulations with respect to having adequate personal insurance coverage (The minimum prescribed liability insurance coverage is as follows:$50,000 for personal injury to, or death of, one person; $100,000 for injury to, or death of, two or more persons in one accident; and $50,000 for property damage.)
- For employees that are not in the DMV pull system, departments should request the employee provide them a copy of their DMV record for review
- Employees must complete the Safe Driver Awareness on-line training (Course Code: DAC-HL0013-SAFSVC).
More About CFR 173.6
CFR 173.6 states that for the transport of clinical samples (that do not harbor Category A materials) the following applies: When transported by motor vehicle in conformance with this section, a material of trade (see §171.8 of this subchapter) is not subject to any other requirements of this subchapter besides those set forth or referenced in this section.
(a) Materials and amounts. A material of trade is limited to the following:
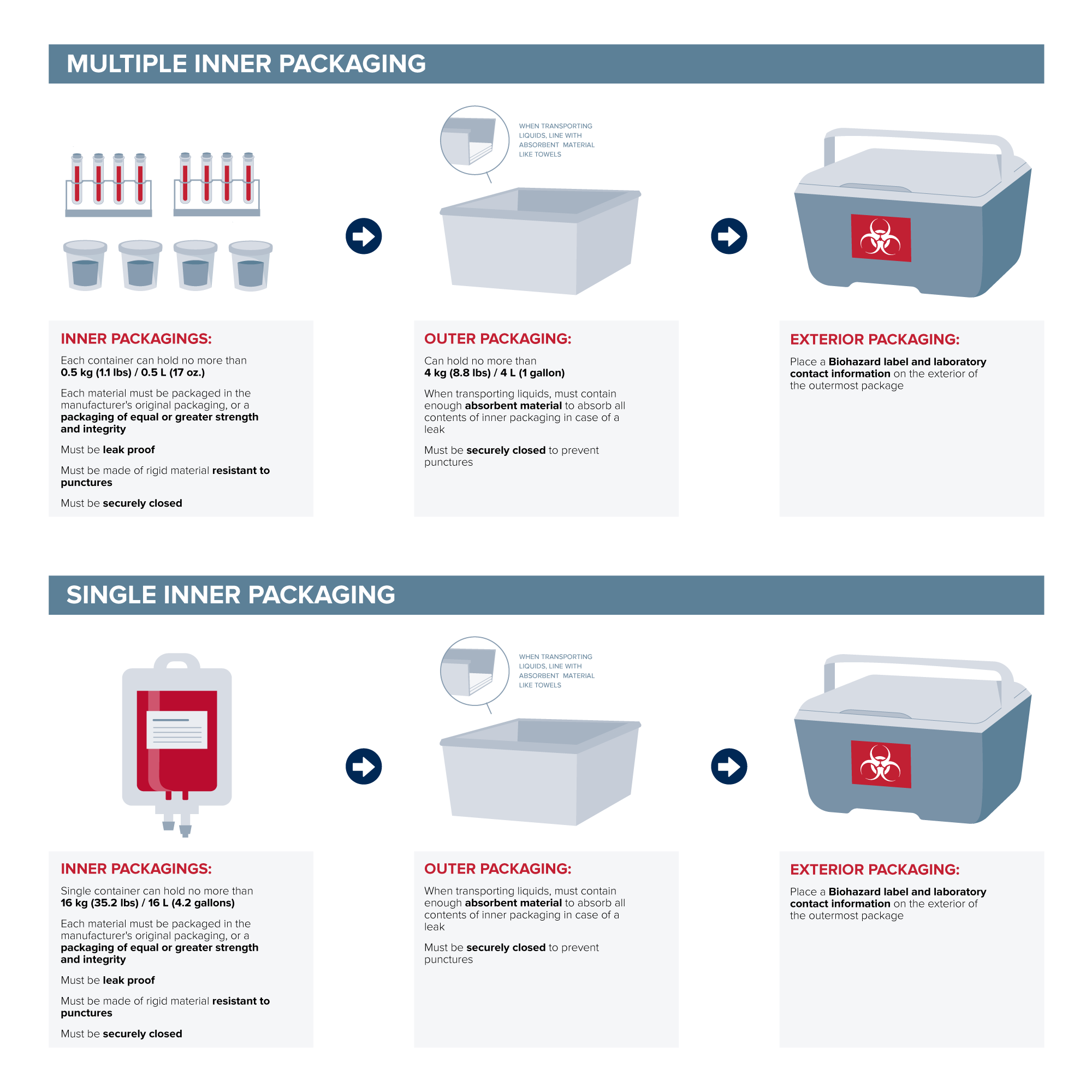
(4) A Division 6.2 material, other than a Category A infectious substance, contained in human or animal samples (including, but not limited to, secreta, excreta, blood and its components, tissue and tissue fluids, and body parts) being transported for research, diagnosis, investigational activities, or disease treatment or prevention, or is a biological product or regulated medical waste. The material must be contained in a combination packaging. For liquids, the inner packaging must be leakproof, and the outer packaging must contain sufficient absorbent material to absorb the entire contents of the inner packaging. For sharps, the inner packaging (sharps container) must be constructed of a rigid material resistant to punctures and securely closed to prevent leaks or punctures, and the outer packaging must be securely closed to prevent leaks or punctures. For solids, liquids, and sharps, the outer packaging must be a strong, tight packaging securely closed and secured against shifting, including relative motion between packages, within the vehicle on which it is being transported.
(i) For other than a regulated medical waste, the amount of Division 6.2 material in a combination packaging must conform to the following limitations:
(A) One or more inner packagings, each of which may not contain more than 0.5 kg (1.1 lbs) or 0.5 L (17 ounces), and an outer packaging containing not more than 4 kg (8.8 lbs) or 4 L (1 gallon); or
(B) A single inner packaging containing not more than 16 kg (35.2 lbs) or 16 L (4.2 gallons) in a single outer packaging.
(6) (b) Packaging…(2) Each material must be packaged in the manufacturer's original packaging, or a packaging of equal or greater strength and integrity.
(c) Hazard communication.……. (4) The operator of a motor vehicle that contains a material of trade must be informed of the presence of the hazardous material (including whether the package contains a reportable quantity) and must be informed of the requirements of this section.
Transport of these human clinical samples are further defined in CFR 173.134 Class 6 Division 6.2.
(b) Exceptions. The following are not subject to the requirements of this subchapter as Division 6.2 materials:
………(10) A Division 6.2 material, other than a Category A infectious substance, contained in a patient sample being transported for research, diagnosis, investigational activities, or disease treatment or prevention, or a biological product, when such materials are transported by a private or contract carrier in a motor vehicle used exclusively to transport such materials*. Medical or clinical equipment and laboratory products may be transported aboard the same vehicle provided they are properly packaged and secured against exposure or contamination. If the human or animal sample or biological product meets the definition of regulated medical waste in paragraph (a)(5) of this section, it must be offered for transportation and transported in conformance with the appropriate requirements for regulated medical waste.
The UC Davis Institutional Biosafety Committee further recommends to adhere to the transportation guidance set forth in the Biosafety in Microbiological and Biomedical Laboratories (see p.133) advisory document, by placing a Biohazard label and contact information on the exterior of the outermost package. These measures would assist in preventing an accidental exposure to a first responder if the vehicle were to be involved in an accident.